TIVUS™ Ultrasound System
Ultimate Standard for Hypertension Treatment
Recognized as no-touch, deepest and fastest Renal Denervation technology
TIVUS system for
renal denervation by SoniVie
CAUTION: Investigational device. Limited by Federal (or United States) law to investigational use. Exclusively for clinical investigation. The safety and effectiveness of renal nerve denervation with the TIVUS System has not yet been established. However, studies have demonstrated the safety and effectiveness of other renal nerve denervation devices for this indication (PMAs P220023 and P220026).

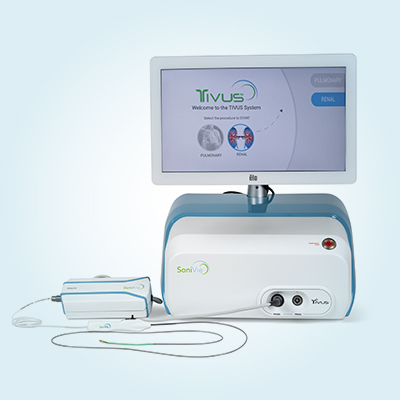
TIVUS
Therapeutic IntraVascular UltraSound
TIVUS is an investigational intravascular device designed for Renal Denervation procedures to treat patients suffering from Hypertension.
Early clinical studies have shown that TIVUS can safely reduce blood pressure in patients suffering from uncontrolled Hypertension.


Founded in 2014 on the achievements of the Therapeutic Intra-Vascular UltraSound-based technology (TIVUS) developed by Cardiosonic for Renal Denervation to treat Hypertension.
SoniVie has been performing clinical trials in both Pulmonary Hypertension and Renal Denervation, has been awarded three Breakthrough Device Designations from the FDA, and has obtained two Premarket Applications for FDA approvals.
SoniVie recently completed the enrollment of a Renal Denervation FDA IDE Pilot Trial and started a global Renal Denervation FDA IDE Pivotal Trial.
Latest News
Boston Scientific announces agreement to acquire SoniVie Ltd.
Acquisition to expand Interventional Cardiology Therapies offerings with ultrasound-based renal denervation therapy for treatment of hypertension MARLBOROUGH, Mass., March 3, 2025 /PRNewswire/ — Boston Scientific Corporation (NYSE: BSX) today announced...
SoniVie Ltd., Appoints Veteran Medtech Executive Raymond W. Cohen as Chairman of its Board of Directors
SoniVie Ltd., a medical device company developing a proprietary renal denervation system to treat hypertension, announced today the appointment of Raymond W. Cohen to serve as its chairman of the...
SoniVie announces renal denervation progress: study results and FDA approval for THRIVE
SoniVie announces RDN pilot results, radial access FIH and FDA pivotal IDE approval, initiating the global THRIVE study in US, Europe and Israel SoniVie, which has developed a novel proprietary...



